Description
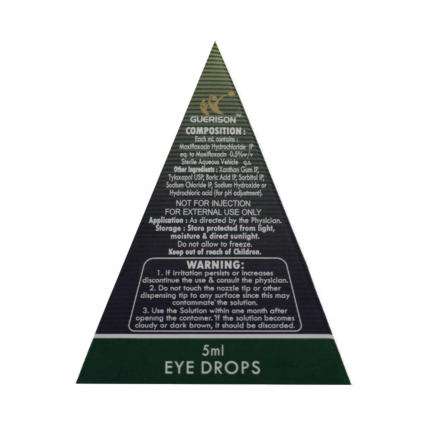
| Composition : | Each ml Contains: – Olopatadine Hydrochloride IP eq. to Olopatadine 0.1% w/v – Benzalkonium Chloride Solution IP 0.01% w/v (As preservative) – Sterile Aqueous Vehicle q.s. |
|---|---|
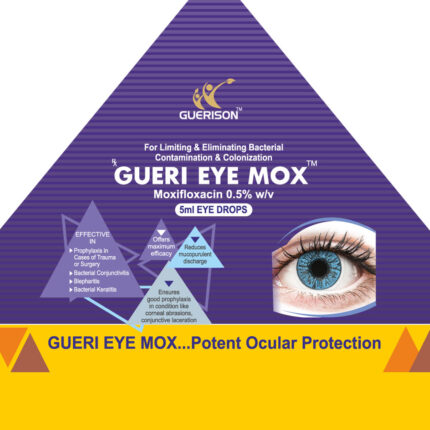
| Advantages: | – Reduce & control the intensity of allergic reactions – Safe and well tolerated in subjects with a history of allergic conjunctivitis – High rates of efficacy in treating ocular itching & offers superior inflammation control |
| Dosage: | As directed by the Physician. |
| Storage: | Store protected from light and moisture, at a temperature between 4°C to 25°C |
| Direction For Use: | – The vial is ready for use when the cap is opened. – Close the cap tightly after every usage. |
| Caution: | Not to be sold by retailers without prescription of registered medical practitioner. |
| Warning: | – If irritation persists or increases discontinue the use and consult your doctor. – Do not touch the nozzle tip to any surface since this may contaminate the suspension. – If particulate matter found, reject the vial and ask for replacement. – Use the suspension within one month after opening the container. |



























Reviews
There are no reviews yet.